Sabine Steffens awarded DFG Funding within the CRC 1744

CRC-1744 | Project A06 | Jürgen Bernhagen and Sabine Steffens: Compartmentalized diversity of inflammatory GPCR networks in neurovascular disease
Vascular inflammation is a major cause of cardiovascular diseases (CVDs). The underlying mecha-nisms have been extensively studied in arterial vascular segments associated with extracranial atherosclerosis. In fact, an increasing number of clinical studies provides proof for the therapeutic utility of anti-inflammatory approaches in atherosclerotic CVDs, but current drug strategies still lack specificity for vascular targets and suffer from immuno¬suppressive side effects.
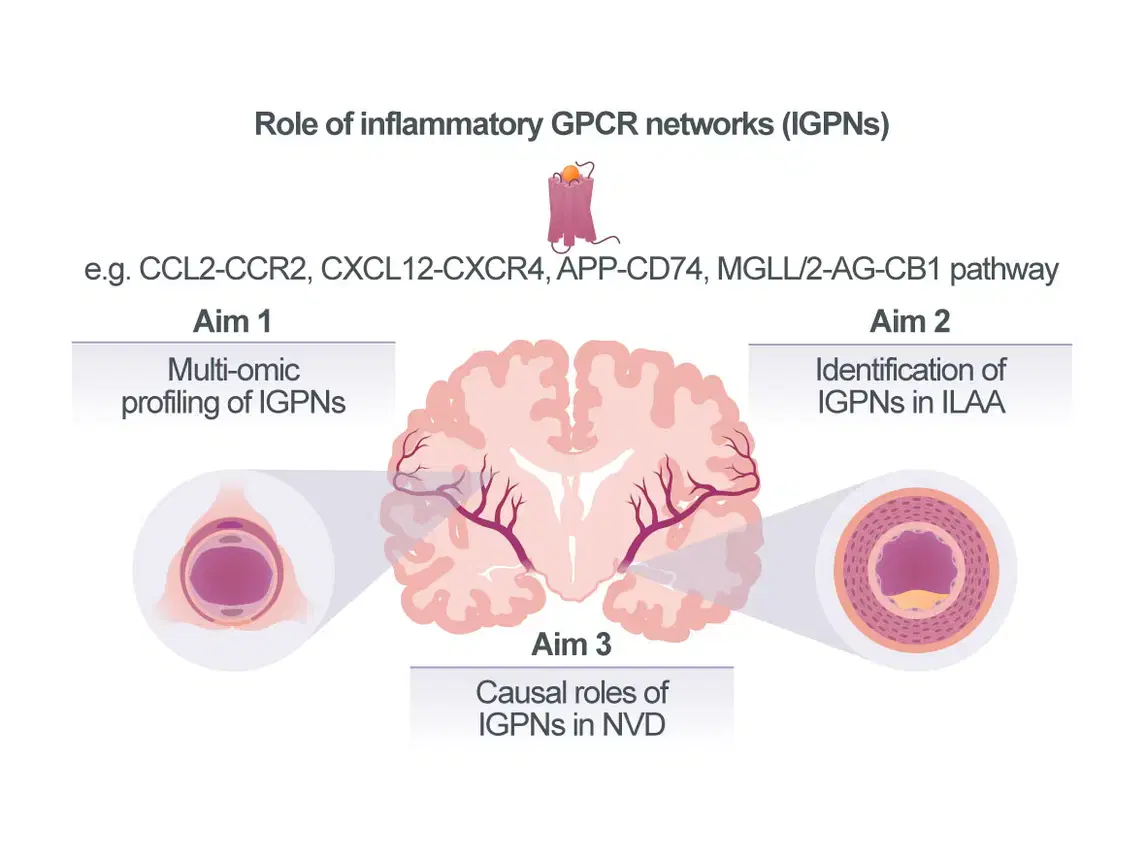
Neurovascular diseases (NVDs) including cerebral small vessel disease (cSVD) and intracranial large-artery atherosclerosis (ILAA) account for a large fraction of strokes and dementias. However, while it is established that the affected vascular segments in extra- vs intracranial vascular disease differ in size and anatomy and despite initial correlative evidence linking inflammation and modifiable cardiovascular risk factors such as obesity, type-2 diabetes (T2D), and hypertension with NVD, the extent and mecha¬nistic understanding of how vascular inflammation drives NVDs is incomplete. Accordingly, treatment options for cSVD and ILAA are currently limited to antithrombotics, antihypertensives, and lipid-reducing drugs, whereas vascular-targeted anti-inflam¬ma¬tory therapies have yet to be established. To this end, chemokines and certain lipid mediators that signal through G protein-coupled receptors (GPCRs), constituting Inflammatory GPCR Networks (IGPNs), represent promising next-generation vascular targets with therapeutic promise. We have extensively studied the atypical chemokine (ACK) MIF, its paralog MIF-2/D-DT, their receptors and the ACK interactome, as well as cannabinoid (lipid)-sensi¬tive receptor networks in athero¬scle¬rosis. These and other studies indicate that IGPNs have key pathogenic roles in atherosclerosis of extra¬cranial arteries, but their contribution to cSVD and ILAA is poorly understood. Further-more, the distri¬bution of IGPNs along the vascular segments associated with NVD is un¬known. We analyzed single-cell-RNAseq data sets from cerebral vasculature and identified cell-specific expression patterns and disease associations of IGPNs, and obtained initial evidence for contributions to cell-communication pathways. Together with preliminary qPCR data from different vascular beds and a receptor knockout (KO) mouse model, this lends support for a compartmentalized role of IGPNs in NVDs.
Our overarching goal is thus to comprehensively profile IGPNs in NVDs and identify candidate IGPN components as potential future therapeutic targets. Capitalizing on tissue, mouse and cell model resources in the CRC, we will first profile IGPNs in cSVD and study the regionality of candidate chemokines, lipid mediators, and their receptors (Aim 1). Furthermore, we will establish experimental in vivo and in vitro models of ILAA and obtain human ILAA biobank specimens to extend IGPN profiling to another neurovascular condition and define IGPN differences between extra- vs intracranial atherosclerotic vascular segments (Aim 2). Pre-identified targets such as CXCL12/CXCR4, MIF/CD74, or the MGLL/2-AG/CB1 axis will be studied for causality by hypo-thesis-driven pharmacological blocking approaches (Aim 3), which will be extended to selected segment-specific chemokines or lipid agonists identified in Aims 1/2. We expect to obtain novel information on IGPNs in cSVD, examine their patterns in different arterial regions to understand their role in ILAA, and explore therapeutic strategies based on identified causal IGPN pathways.